Committed to quality
Committed to quality
Committed to quality
Preparation of reference standards for the visual inspection of parenteral pharmaceuticals
During the production of pharmaceuticals, undesirable defects or particles can occur in or on the product containers. The visual inspection of containers such as ampoules, cartridges, syringes, injection vials, infusion bags, vials, or BFS containers is therefore an essential part in the production process of liquid and freeze-dried pharmaceuticals. Defined reference standards must be used to determine the detection rate of foreign particles and product and container defects during the manual or mechanical visual quality control.
For this purpose, the Material Analytic Service (M.A.S…) GmbH prepares customer specific reference standard test sets for the visual inspection of parenteral pharmaceuticals. The defective particle samples, individually defined by the customer, are prepared and documented in detail by M.A.S…
Liquids
M.A.S… creates reference standard test kits for liquid parenteral pharmaceuticals
Lyophilisates
M.A.S… creates reference standard test kits for lyophilized pharmaceuticals
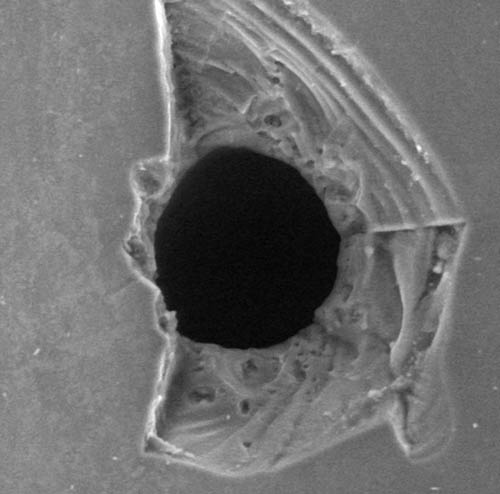
Pinholes
M.A.S… creates pinholes for container seal integrity tests of different containers
Media fill
M.A.S… creates test kits for the simulation of microbiological contamination
Liquids
M.A.S… creates reference standard test kits for liquid parenteral pharmaceuticals
Lyophilisates
M.A.S… creates reference standard test kits for lyophilized pharmaceuticals
Liquids
M.A.S… creates reference standard test kits for liquid parenteral pharmaceuticals